12c. Anatomy and Physiological aspects of Puberty and Menopause
Unit 12C: Anatomy and Physiological Aspects of Puberty and Menopause
Learning Goals
By the end of this chapter, you should be able to:
- describe the anatomical structures and maturational changes of the hypothalamic–pituitary–ovarian (HPO) axis, ovaries, uterus, breasts and external genitalia at puberty and menopause;
- explain the physiology of pubertal onset (adrenarche, gonadarche, menarche) and menopausal transition (follicular depletion, neurovascular instability);
- identify clinical features, investigations and first-line management principles for disorders of puberty and menopause, linking these to underlying physiology;
- apply integrative reasoning using Ayurvedic terms (ṛtuśuddhi, rajonivṛtti) while retaining modern anatomical–physiological precision.
1) Puberty: Anatomical Foundations
Axis components. Puberty emerges from coordinated maturation of:
- Hypothalamus (arcuate and preoptic areas) that generates pulsatile GnRH;
- Anterior pituitary (gonadotrophs) that secrete LH/FSH;
- Ovaries, where theca and granulosa cells produce oestrogens, progesterone, inhibin/activin;
- End organs—uterus, cervix, vagina, breasts, external genitalia, skeleton and CNS.
Pelvic organ growth. Oestrogen causes:
- Uterine enlargement: from infantile (~3.5 cm, cervix>body) to pubertal/adult (~6–8 cm, body>cervix). Endometrium acquires proliferative–secretory cycling.
- Ovarian growth: increased stromal volume; appearance of multiple antral follicles.
- Vaginal maturation: epithelium becomes oestrogenised, multilayered, with glycogen; pH turns acidic.
- Breast development (thelarche): lobuloalveolar growth, ductal branching; areolar enlargement.
- External genitalia: labia majora/minora hypertrophy; pubarche reflects adrenal androgens.
Skeletal and body composition. Oestrogen drives epiphyseal maturation and closure, producing the peak height velocity (PHV) just before menarche, alongside increase in fat mass (particularly gynoid deposition).
Tanner Sexual Maturity Staging (SMR)
| Feature | SMR 1 | SMR 2 | SMR 3 | SMR 4 | SMR 5 |
|---|---|---|---|---|---|
| Breast | Preadolescent | Breast bud | Further enlargement | Secondary mound | Mature contour |
| Pubic hair | None | Sparse, straight | Coarser, dark, spread | Adult-type limited area | Adult in quantity & spread |
2) Puberty: Physiological Milestones
Two preparatory processes precede regular ovulation:
- Adrenarche (≈6–8 years): zona reticularis matures → DHEA/DHEAS rise → pubarche, axillary hair, body odour. It is adrenal, not gonadal.
- Gonadarche (≈8–13 years): hypothalamic KNDy (Kisspeptin–Neurokinin B–Dynorphin) neurons establish nocturnal, then diurnal GnRH pulses. Pituitary LH/FSH increase; ovaries produce oestrogens.
Sequence to menarche (typical):
Thelarche → Pubarche → PHV → Menarche (about 2–2.5 years after thelarche). The first 1–2 years after menarche are often anovulatory because positive feedback (LH surge generation) is still maturing.
Cycle maturation. Early post-menarche cycles may be irregular, long (immature follicular phase), or occasionally short; progressively the follicular phase standardises, ovulation becomes regular, and luteal progesterone achieves consistent endometrial transformation.
Physiological regulators.
- Energy signals: leptin (adequate fat stores) permits pulsatility; severe caloric deficit or over-exercise suppresses the axis (functional hypothalamic amenorrhoea).
- Thyroid and adrenal milieu: euthyroid status and moderate cortisol favour normal timing; chronic stress can delay or disrupt cycles.
- Chronobiology: GnRH pulses are initially nocturnal; sleep deprivation distorts timing.
3) Puberty: What Changes Are You Expected to Spot Clinically?
- Breasts: symmetrical, sometimes tender; transient asymmetry is common.
- Growth: the PHV precedes menarche; after menarche, only ~5–7 cm further height gain.
- Genital tract: leukorrhoea may appear with oestrogenisation; reassure if non-offensive.
- Psychosocial: mood variability reflects neurosteroid effects; address nutrition, sport, sleep and menstrual education.
Disorders to recognise early
- Precocious puberty: secondary sexual characters before 8 years; evaluate for central (GnRH-dependent) vs peripheral causes.
- Delayed puberty/primary amenorrhoea: no thelarche by 13 or no menarche by 15 (or 3 years post-thelarche); map along the HPO pathway and outflow tract.
4) Menopause: Anatomical Changes
Definition. Menopause is the final menstrual period after 12 months of amenorrhoea not due to other causes. The transition (perimenopause) spans variable years before and after this point.
Ovaries. Lifelong follicle attrition accelerates in the late 30s; when the pool nears exhaustion, oocyte/follicle number and granulosa function fall. Anatomically, ovaries shrink (often <2–3 cm), surface becomes smoother with fewer dominant follicles.
Uterus and cervix. With hypoestrogenism:
- Uterus becomes smaller; myometrium thins; endometrium atrophies unless exogenous hormones are used.
- Cervix reduces in size; external os may narrow; cervical mucus becomes scant.
Vagina and vulva. Genitourinary syndrome of menopause (GSM) encompasses:
- Vaginal epithelium thinning, loss of rugae, reduced glycogen → ↑ pH, dryness, dyspareunia.
- Vulvar thinning with fissuring in severe cases; urethral mucosa atrophy contributes to frequency/urgency/dysuria.
Breasts. Involution—lobules shrink; fat replaces gland; stromal density decreases (mammographic changes).
Skeleton and body composition. Rapid bone loss (first 3–5 years) from accelerated osteoclast activity; sarcopenia increases; central (visceral) adiposity rises.
5) Menopause: Physiological Transitions
Hormonal profile.
- Estradiol (E2) declines; estrone (E1) from peripheral aromatisation predominates.
- Progesterone is low due to anovulation.
- Inhibin B/A fall early → FSH rises (FSH > LH), a hallmark of ovarian insufficiency.
- AMH approaches zero (reflects follicle pool).
- Androgens (ovarian/adrenal) decline more gradually; relatively higher androgen:oestrogen ratio may produce hirsutism in some women.
Vasomotor symptoms (VMS).
Hot flushes and night sweats arise from hypothalamic thermoregulatory set-point narrowing. Oestrogen deficiency alters KNDy neuron activity (↑ neurokinin B signalling), making minor core temperature changes trigger heat-dissipation responses (flush/sweat).
Metabolic and vascular changes.
Loss of oestrogen’s vascular protection increases cardiometabolic risk (lipids, endothelial function, insulin sensitivity). Central adiposity and sleep disruption (from VMS) amplify this risk.
Neurocognitive and mood.
Some experience sleep fragmentation, reduced concentration and low mood, linked to VMS and steroid withdrawal. Major depressive disorder is not inevitable; screen if symptoms persist.
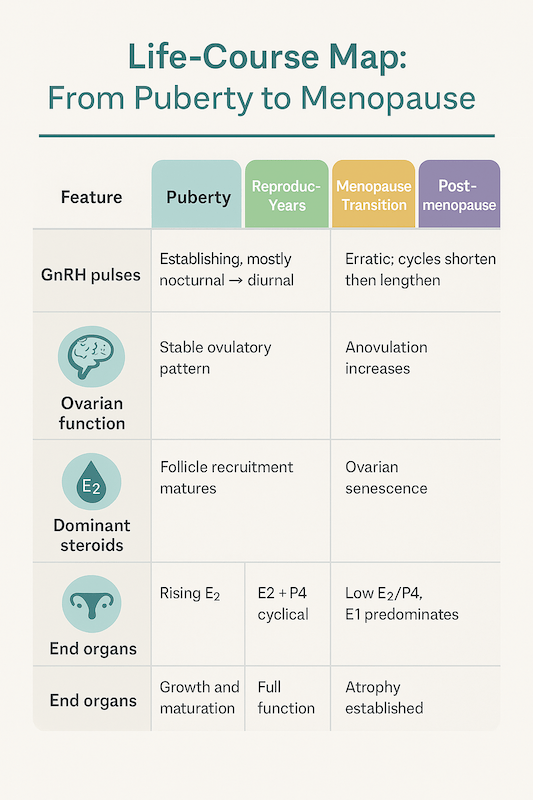
6) Life-Course Map: From Puberty to Menopause
| Feature | Puberty | Reproductive Years | Menopause Transition | Postmenopause |
|---|---|---|---|---|
| GnRH pulses | Establishing, mostly nocturnal → diurnal | Stable ovulatory pattern | Erratic; cycles shorten then lengthen | Low gonadotropin response despite high FSH/LH |
| Ovarian function | Follicle recruitment matures | Regular ovulation | Anovulation increases | Ovarian senescence |
| Dominant steroids | Rising E2 | E2 + P4 cyclical | Falling E2/P4 | Low E2/P4, E1 predominates |
| End organs | Growth and maturation | Full function | Atrophy begins | Atrophy established |
7) Standardised Staging: STRAW+10
| Stage | Label | Clinical cues |
|---|---|---|
| -2 | Early menopausal transition | Cycle variability ≥7 days compared with usual |
| -1 | Late transition | ≥60 days amenorrhoea; VMS common |
| 0 | Final Menstrual Period (FMP) | Defined retrospectively after 12 months |
| +1a–c | Early postmenopause (first 5 years) | VMS peak; rapid bone loss |
| +2 | Late postmenopause | Genitourinary changes dominate; stable low hormones |
8) Investigations—Physiology Guides the Choice
Puberty concerns
- Precocious puberty: bone age, basal and GnRH-stimulated LH/FSH, pelvic ultrasound; MRI brain if central precocity suspected.
- Delayed puberty/primary amenorrhoea: FSH/LH/E2, thyroid profile, prolactin; pelvic ultrasound (uterus/ovaries), karyotype where indicated; screen for chronic illness/nutrition.
Menopause and perimenopause
- Typical age/symptoms: diagnosis is clinical; routine hormone testing is usually unnecessary.
- If uncertain or premature ovarian insufficiency (POI) suspected (<40 years): FSH twice, 4–6 weeks apart, elevated; E2 low, AMH very low.
- Evaluate lipids, glucose, thyroid as baseline health screens; ensure bone mineral density testing if risk factors or after FMP with symptoms.
9) Management Principles
A. Puberty
- Normal variation: reassure; teach menstrual literacy, nutrition (iron, calcium, vitamin D), sleep hygiene and sport.
- Functional hypothalamic amenorrhoea: restore energy balance; reduce training load; psychological support.
- Central precocious puberty: GnRH agonists suppress axis until appropriate age to preserve height and psychosocial well-being.
- Peripheral precocity: treat source (ovarian/adrenal tumour, exogenous hormones).
- Delayed puberty/primary amenorrhoea: treat cause—thyroid disease, coeliac, hyperprolactinaemia, POI; induce puberty gradually when indicated.
B. Menopause
- Lifestyle: weight management, resistance + impact exercise (bone), sleep regularity, limit alcohol, stop smoking.
- Menopausal Hormone Therapy (MHT):
- Indication: moderate–severe VMS, early menopause/POI, bothersome GSM, bone protection in symptomatic women <60 years or within 10 years of FMP, after risk assessment.
- Regimens:
- Uterus present: oestrogen + progestogen (sequential if <12 months amenorrhoea; continuous combined after).
- Post-hysterectomy: oestrogen-only.
- Local vaginal oestrogen for GSM (minimal systemic absorption).
- Contraindications (absolute): active or history of breast cancer, oestrogen-dependent malignancy, unexplained vaginal bleeding, active VTE or thrombophilia, active liver disease.
- Relative: migraine aura, high CVD risk—consider transdermal routes and lowest effective dose.
- Non-hormonal options: SSRIs/SNRIs, gabapentin, clonidine for VMS when MHT is contraindicated or declined.
- Bone health: calcium (diet first), vitamin D sufficiency, exercise; bisphosphonates or denosumab if osteoporosis/fracture risk high.
- Urogenital: vaginal oestrogen, moisturisers; pelvic floor exercises; manage recurrent UTIs per protocol.
- Cardiometabolic: screen BP, lipids, glucose; manage risk factors early.
10) Integrative Notes
While puberty and menopause are framed here with modern physiology, their clinical essence resonates with Ayurveda:
- Puberty parallels restoration of ṛtuśuddhi (appropriate cyclicity) supported by āhāra–vihāra balance. States of alpāhāra (caloric deficit), ati-vyāyāma (excess exercise) and mano-duṣṭi (stress) mirror hypothalamic suppression.
- Menopause corresponds to rajonivṛtti (cessation of menses) with emphasis on maintaining dhātu-balā (tissue strength)—bone (asthi), mind (manas), and agni stability through tailored diet, sleep and movement.
(Direct śloka citation is not essential here because this is primarily a modern anatomy–physiology topic; you will find exact ślokas with references in classical pathya–apathya and cikitsā chapters.)
11) Ten High-Yield Revisions
- Thelarche → Pubarche → PHV → Menarche is the usual sequence.
- Early post-menarche cycles are often anovulatory; this normalises within ~2 years.
- Leptin and energy sufficiency permit GnRH pulsatility; severe deficits suppress it.
- Pubertal uterus: body>cervix; infant uterus: cervix>body.
- Menopause: FSH rises early as inhibin falls; E2 declines, E1 predominates.
- KNDy neuron changes explain vasomotor symptoms.
- GSM results from genital tract atrophy and higher vaginal pH; treat with local oestrogen.
- MHT works best when started <60 years or within 10 years of FMP, after risk assessment.
- In POI, hormone therapy is recommended till average menopausal age for bone/cardiovascular protection.
- Always distinguish central vs peripheral precocity and HPO vs outflow causes of amenorrhoea.
Assessment
A) MCQs (one best answer)
- The first endocrine event that typically heralds puberty in girls is:
A. Gonadarche B. Adrenarche C. Menarche D. Peak height velocity
Answer: B - The usual order of pubertal milestones is:
A. Pubarche → Menarche → Thelarche → PHV
B. Thelarche → PHV → Pubarche → Menarche
C. Thelarche → Pubarche → PHV → Menarche
D. PHV → Thelarche → Pubarche → Menarche
Answer: C - In the pubertal uterus, the proportion of cervix to body becomes:
A. Cervix>body B. Body>cervix C. Equal D. No change from infancy
Answer: B - The hormone most responsible for epiphyseal closure in girls is:
A. Growth hormone B. Oestradiol C. Progesterone D. Cortisol
Answer: B - The earliest biochemical marker to rise as the menopausal transition begins is:
A. Estradiol B. Progesterone C. Inhibin D. FSH
Answer: D (FSH rises as inhibin falls) - Hot flushes are best explained by:
A. Hyperthyroidism
B. Narrowing of hypothalamic thermoneutral zone via KNDy changes
C. Elevated prolactin
D. Increased cortisol
Answer: B - A 47-year-old has cycle variability >7 days for the last year and night sweats. STRAW+10 stage is:
A. -2 (early transition) B. -1 (late transition) C. 0 (FMP) D. +1
Answer: A - Which of the following is an absolute contraindication to systemic MHT?
A. Controlled hypertension B. Prior VTE (active thrombophilia) C. Osteopenia D. Atrophic vaginitis
Answer: B - A 12-year-old, 2 months post-menarche, has 45-day cycles. The most likely explanation is:
A. Thyroid disease B. Physiological anovulation in early post-menarche years C. PCOS D. Hyperprolactinaemia
Answer: B - The most appropriate first-line therapy for GSM with dyspareunia in a healthy postmenopausal woman is:
A. Systemic MHT B. Vaginal oestrogen C. SSRI D. Clonidine
Answer: B
B) Short Answer Questions (3–5 lines each)
- Outline the anatomical differences between the infantile and pubertal uterus and cervix.
- Explain the roles of adrenarche and gonadarche in female puberty.
- List three physiological mechanisms for vasomotor symptoms in menopause.
- Enumerate absolute contraindications to systemic menopausal hormone therapy.
- Define premature ovarian insufficiency and name two investigations that support the diagnosis.
C) Long Answer Questions
- Describe the anatomical and physiological changes during female puberty, including HPO maturation, skeletal effects, genital tract changes and sequence to menarche. Discuss common deviations (precocious, delayed puberty) with first-line evaluation.
- Explain menopausal physiology and anatomy—ovarian senescence, hypothalamic thermoregulation, GSM, bone and cardiovascular changes. Outline evidence-based indications, regimens, and contraindications for MHT, and non-hormonal options.
D) Case-Based (OSCE-style)
A 49-year-old teacher has 3 months of amenorrhoea after years of 26–28 day cycles, with hot flushes disturbing sleep.
- a) Assign a STRAW+10 stage based on her history.
- b) List two lifestyle and two pharmacological options for vasomotor relief.
- c) What baseline health checks will you offer before starting therapy?
